From October 31st to November 2nd, 2024, the Spobiotic Research Center, ANABIO R&D, participated in the 25th National Pediatrics Conference in Hue City entiled “From Science to Policy and Practice.” The conference is a prestigious scientific forum, gathering leading experts from Vietnam and abroad to share the latest advancements in pediatrics.
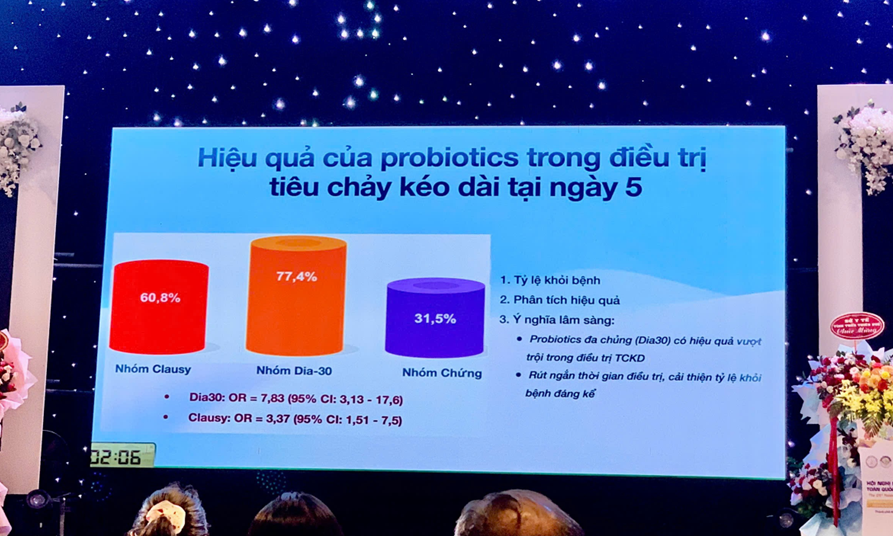
At the conference, Dr. Dang Thuy Ha, MD, PhD, Deputy Head of the Gastroenterology Department at the National Children’s Hospital, presented the report “Characteristics of Gut Microbiota and Treatment Outcomes for Persistent Diarrhea at the National Children’s Hospital.” This research was conducted in collaboration between the doctors of the National Children’s Hospital and the Spobiotic Research Center, ANABIO R&D. The report highlighted positive results in shortening treatment duration and increasing the effectiveness of managing persistent diarrhea in children by combining conventional treatments with water-based probiotic Bacillus spore products such as LiveSpo CLAUSY (Bacillus clausii, 2 billion CFU/5 mL) and LiveSpo DIA30 (Bacillus subtilis, Bacillus clausii, Bacillus coagulans, 5 billion CFU/5 mL). These products have been recognized as promising solutions in treating prolonged diarrhea, contributing to reduced antibiotic use in children. Findings from the study were published in Nature Scientific Reports in March 2024, providing further scientific support for optimizing treatment protocols for children with prolonged diarrhea.
The conference also featured participation from numerous health administrators and leaders from healthcare facilities across the country, offering an opportunity for a comprehensive evaluation of issues in pediatrics to develop and refine appropriate health policies. Contributions at the conference not only aim to enhance the quality of child healthcare but also lay the foundation for sustainable development of Vietnam’s healthcare sector in pediatrics. This information serves as valuable updates for scientists of the Spobiotic Research Center, ANABIO R&D, to generate ideas for probiotic product development, aligned with the mission of “a future without antibiotics.”