Ngày 18/05/2025 đánh dấu cột mốc 3 năm hình thành và phát triển của Trung tâm Nghiên cứu Bào tử Lợi khuẩn (Spobiotic Research Center) – đơn vị tiên phong tại Việt Nam trong nghiên cứu và ứng dụng công nghệ bào tử lợi khuẩn vì sức khỏe cộng đồng. Với sứ mệnh “Vì tương lai không kháng sinh”, Trung tâm đã không ngừng đổi mới và nỗ lực phát triển các sản phẩm sinh học an toàn, hiệu quả phục vụ y tế dự phòng và hỗ trợ điều trị.
Những thành tựu nổi bật sau 3 năm hoạt động:
- Nghiên cứu và thử nghiệm lâm sàng:
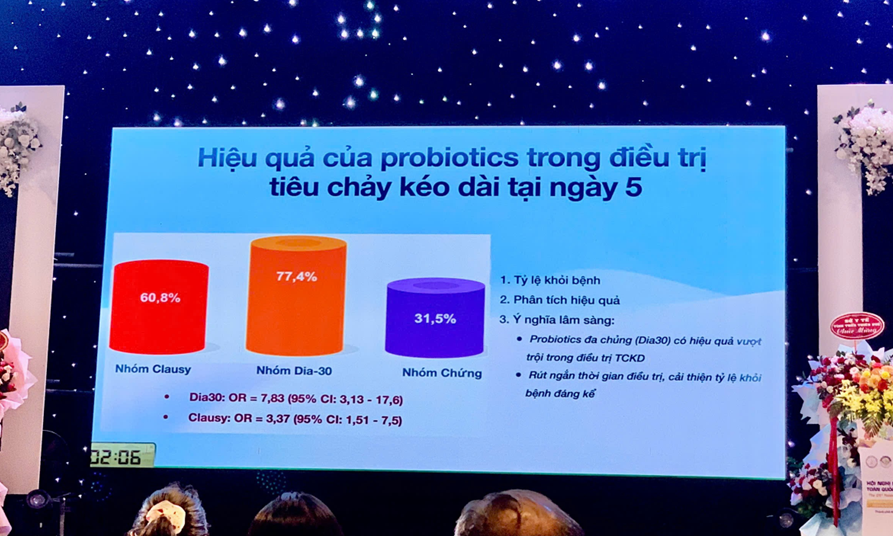
Trung tâm đã phối hợp triển khai nhiều đề tài nghiên cứu và thử nghiệm lâm sàng với các sản phẩm tiêu biểu như LiveSpo® NAVAX (4 đề tài), LiveSpo® PREG-MOM & KIDS (1 đề tài), LiveSpo® CLAUSY & DIA30 (1 đề tài), và LiveSpo® X-SECRET (2 đề tài). Các nghiên cứu đã cung cấp bằng chứng khoa học về độ an toàn và hiệu quả của bào tử lợi khuẩn Bacillus trong hỗ trợ điều trị các bệnh lý hô hấp, tiêu hóa và phụ khoa. - Công bố khoa học:
Đã có hơn 9 bài báo quốc tế, trong đó có 4 bài đăng trên tạp chí Nature Scientific Reports, cùng với 15 bài báo trong nước và 12 báo cáo hội nghị khoa học. Những công bố này góp phần khẳng định năng lực nghiên cứu và vị thế học thuật của Trung tâm trên trường khoa học quốc tế. - Sở hữu trí tuệ:
Trung tâm đã đăng ký thành công 5 sáng chế liên quan đến quy trình sản xuất các sản phẩm chủ lực như LiveSpo COLON, NAVAX, X-SECRET, cùng 2 sản phẩm thế hệ mới. Ngoài ra, đã bảo hộ thành công 8 nhãn hiệu độc quyền, thể hiện tính sáng tạo và định hướng phát triển bền vững trong các lĩnh vực tiêu hóa, hô hấp và phụ khoa. - Hợp tác trong nước và quốc tế:
Trung tâm mở rộng mạng lưới hợp tác với nhiều bệnh viện và đơn vị nghiên cứu như Bệnh viện Nhi Trung ương, Bệnh viện Phụ sản Hà Nội, ĐH Y Hà Nội, ĐH Y Dược Thái Bình, ĐH Kyoto, ĐH Tohoku, Viện Dinh Dưỡng, CDC Bắc Ninh… Từ đó từng bước khẳng định vai trò tiên phong của Việt Nam trong nghiên cứu bào tử lợi khuẩn.
Hơn cả những con số, chúng tôi tự hào vì mỗi công trình nghiên cứu, mỗi sản phẩm ra đời đều mang trong mình một sứ mệnh: “Vì tương lai không kháng sinh”
Trung tâm xin gửi lời tri ân sâu sắc tới toàn thể đội ngũ nghiên cứu viên, lãnh đạo, các phòng ban, đối tác và cộng sự đã đồng hành trong suốt chặng đường vừa qua.