The 31st Annual National Gastroenterology Scientific Conference (2025), held in Ninh Binh from October 31 to November 1, brought together leading experts to discuss the latest advances in the diagnosis and treatment of gastrointestinal diseases in Vietnam. This year, the conference focused on two major challenges: the increasing prevalence of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) in younger populations, and the persistently high rate of Helicobacter pylori (HP) infection accompanied by rising antibiotic resistance. A common thread connecting these conditions is the essential role of the gut microbiome, making probiotics one of the most prominent topics of discussion at the conference.
Multiple scientific presentations highlighted the rapid rise of IBS and IBD among younger individuals in Vietnam, underscoring the need for safe and long-term supportive therapies. According to Assoc. Prof. Dr. Tran Thi Khanh Tuong, spore-forming Bacillus probiotics and beneficial yeasts show promising potential in reducing inflammation, modulating the gut–brain axis, and improving persistent digestive symptoms. Additionally, given Vietnam’s high HP infection rates and difficulties in managing reinfection, incorporating probiotics into treatment regimens is increasingly viewed by clinicians as a valuable adjunctive approach. However, despite abundant international evidence, Vietnam still lacks high-quality clinical studies conducted directly on the local population.
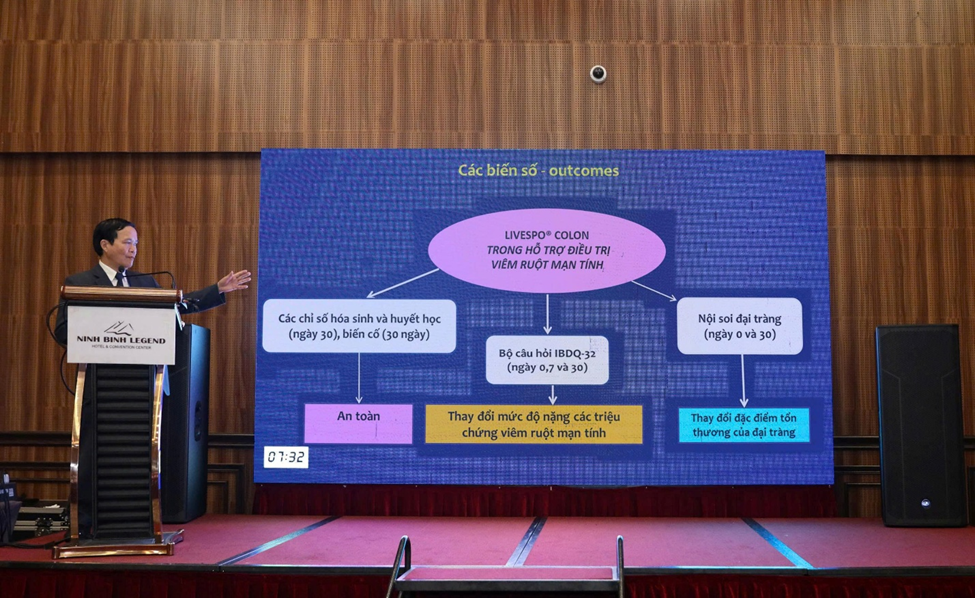
One of the highlights of the conference was the presentation by Assoc. Prof. Dr. Vu Van Khien, Vice President of VNAGE, titled “The role of probiotic in inflammatory bowel diseases: Overview and preliminary finding in Vietnam.” The report introduced a study conducted at Thai Binh University of Medicine and Pharmacy evaluating a multi-strain Bacillus formulation in the product LiveSpo Colon among IBD patients. The results demonstrated a favorable safety profile, with no serious adverse events recorded during the 30-day follow-up and no abnormalities in subclinical laboratory parameters. Clinical symptoms of IBD—including abdominal pain, diarrhea, constipation, mucus or bloody stools, fatigue, and weight loss—showed significant improvement at both day 7 and day 30 in the LiveSpo Colon group, with greater improvement compared to the control group. Colonoscopy findings after 30 days also revealed clearer mucosal recovery, suggesting the supportive therapeutic potential of the Bacillus formulation.
Against the backdrop of the challenges highlighted by leading experts, LiveSpo’s vision of “A future without antibiotics” was emphasized as a forward-looking strategy aligned with global trends. Investment in clinical research, the development of robust Bacillus spores, and a microbiome-centered approach reflect LiveSpo’s commitment to providing sustainable solutions for public health. This direction represents not only scientific advancement but also social responsibility in the face of the growing threat of antibiotic resistance.

Overall, this year’s conference reflected a clear shift in Vietnam’s gastroenterology field—from a purely drug-centered approach to strategies focused on microbiome modulation and personalized care. Probiotics, especially Bacillus-based formulations, are emerging as promising adjunctive options for various gastrointestinal disorders. However, for probiotics to be formally integrated into clinical practice in Vietnam, high-quality local studies remain essential. During this transition, the contributions of research institutions and pioneering companies, such as LiveSpo, play a significant role in generating evidence and drawing attention from clinicians and experts nationwide.