On the morning of February 10, 2025, the Spobio Research Center was honored to welcome Mrs. Isabelle Ducellier, the former CEO of Biogaia, for a visit and consultation regarding the potential development of probiotic products for the international market. This meeting provided a valuable opportunity to share ANABIO R&D’s research advancements and receive expert insights from a leading specialist in the field.
The 60-minute exchange left a profound impression on Mrs. Isabelle Ducellier, owing to the outstanding research conducted by the R&D laboratory. The collaborations with prestigious institutions, including the Central Pediatric Hospital, Thai Binh University of Medicine and Pharmacy, and the National Institute of Nutrition, have contributed to documenting the tangible benefits of probiotics in supporting the treatment of respiratory, digestive, and various other health conditions.
During the meeting, the research team presented their flagship probiotic products: PregMom, KID, Dia30, Colon, Clausy, Navax, and X-secret. These products have demonstrated exceptional efficacy in clinical trials and have been featured in renowned scientific journals such as Nature Scientific Reports and ASM. Notable findings include:
LiveSpo Navax effectively alleviates symptoms of ARTI caused by RSV while reducing viral load, offering a simple, cost-effective treatment approach for viral infections in general.
LiveSpo Colon significantly improves symptoms of chronic intestinal inflammation, including colitis, diarrhea, and constipation, while enhancing inflammatory markers and quality of life after 30 days of treatment. These improvements have been documented through comparative colonoscopy images from before and after product use.
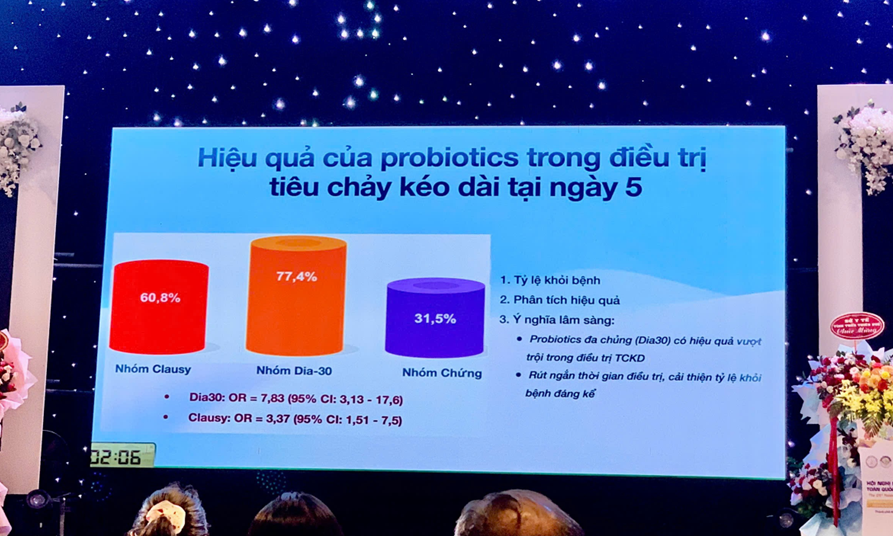
LiveSpo Clausy regulates inflammation through the Th17 mechanism and has shown particularly promising results in reducing the duration of prolonged diarrhea in children aged 3-24 months. This contributes to reducing antibiotic use, protecting gut microbiota, and promoting healthier growth in children.
LiveSpo X-secret effectively alleviates symptoms of STDs, reduces pathogenic bacterial load, and improves the vaginal microbiota.
LiveSpo DIA30 has demonstrated remarkable effectiveness in treating prolonged diarrhea in children by reducing inflammation and improving gut microbiota without requiring antibiotics.
LiveSpo KIDS and LiveSpo PREG-MOM effectively alleviate constipation and malnutrition in children while strengthening immune systems and restoring gut microbiota.
Significantly, all ANABIO probiotic products have proven safe, with no reported side effects in clinical studies. This evidence demonstrates the effectiveness of probiotics in reducing antibiotic treatment duration, aligning with ANABIO R&D’s overarching goal: “For a future without antibiotics.”
In addition to the achievements, Mrs. Isabelle Ducellier was particularly impressed by the community-focused vision that ANABIO’s founder has instilled in every member of the research team. The unity, relentless creativity, and determination to overcome challenges in completing each study clearly reflect their shared goal: to benefit public health, with a particular focus on children’s health.
This meeting not only provided an opportunity for the Spobio Research Center to showcase its scientific accomplishments to an international expert but also served as a testament to ANABIO R&D’s commitment to LiveSpo Pharma’s vision: “For a future without antibiotics.”
Here are some pictures from the tour